No one could foresee the impact that the Covid-19 outbreak would have on the different processes we take for granted. The pharmaceutical industry in particular is facing major disruption, as clinical trials have come into contact with unfamiliar challenges. With no further assurance that another pandemic will not hit again, the pharmaceutical industry should take this outbreak as an opportunity to find alternatives to set processes. This will enable them to handle similar uncertainties in the future with ease.
Industry leaders such as Bristol Myers Squibb and Eli Lilly have shared the extent of Covid-19’s impact on certain ongoing trials. Other mid-pharmacos such as Vertex Pharmaceuticals and clinical stage biotech companies like Moderna are facing similar challenges.
The management of clinical trials already comes with multifaceted issues, however new challenges have surfaced such as:
- Differential lock-down periods across geographies that have added to the existing pressures in managing trial sites.
- Conducting clinical trials during quarantine, as both patients and personnel are unavailable for in-person visits.
- The increasing enrollment of new patients due to this infection could skew the outcome of results.
- Multispecialty hospitals must dedicate large parts of their facilities for Covid-19 patients’. Such hospitals in case acting as clinical trial sites, are putting participating patients in clinical trials at risk of acquiring the infection.
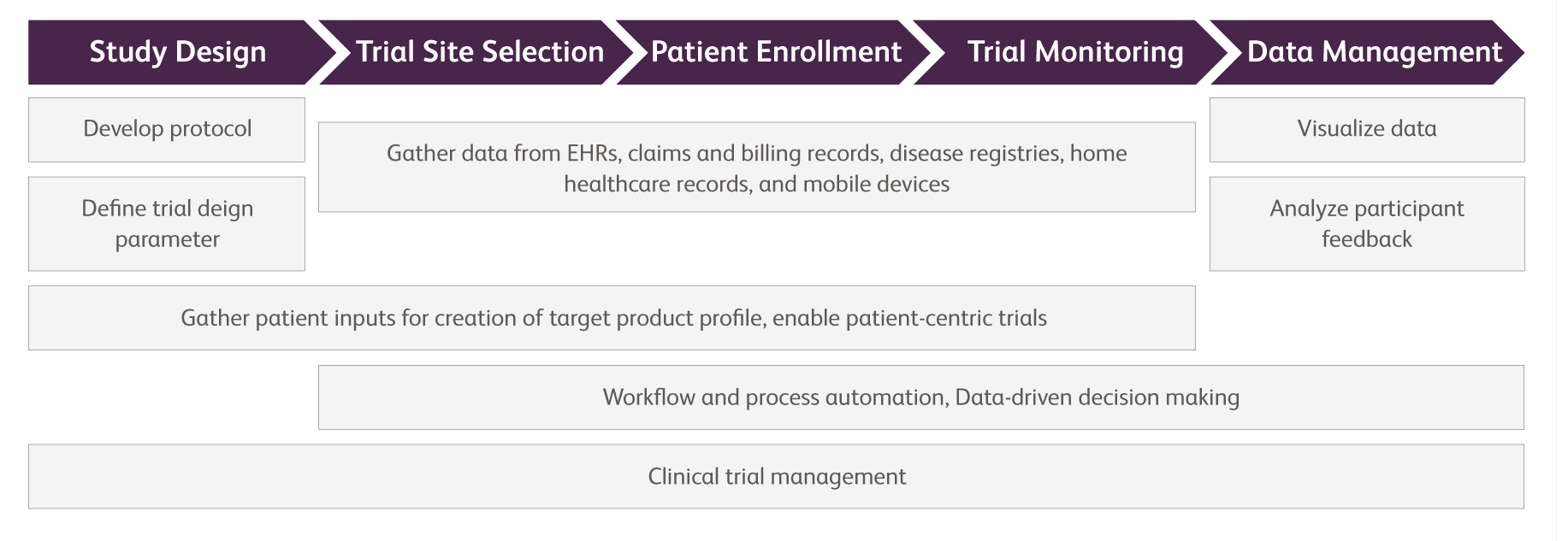
Clinical trials are not optional, and they are the central mechanism for unbiased evaluation of advances in healthcare and comparative analysis of diagnosis, treatment and management options. Turning to technology and digital innovation is undoubtedly a solution to combat the mayhem. The Covid-19 outbreak is pushing for the future to happen now. ‘Digital clinical trials’ which was a mere concept previously, is not on-track to become a reality.
Across the executional elements of clinical trials, digitalization and effective use of technologies such as machine learning, and artificial intelligence can play a part in resolving major issues. Many of the issues will experience resolutions leading to reduction in repetitive tasks at clinical trial sites, data standardization, higher participation of patients (lower reluctance of patients to travel to trial sites), increased diversity of patient enrollment (no location constraint), and lowered cost of conducting clinical trials.
However, transitioning into digital processes is easier said than done, as this transition will entail more than just changing from paper to digital mediums. Data privacy has been a persistent issue with the current digital revolution in other industries, and directly applies to clinical research as well. In addition, the sheer quantity of research data poses a problem, due to clinical research data being sparred across various research institutes. Even when data is stored within one institute, the data is still siloed internally, which makes it difficult for information to be shared.
Becoming a part of the solution
There are different ways that organizations can start to adapt their clinical processes for a post-Covid world, such as:
- Establishing participant data transparency and privacy. It will be critical to establish standard processes that support the transparency and privacy of a participant’s data. This has already started with companies who comply to regulations, such as the General Data Protection Regulation (GDPR) in the EU and the California Consumer Privacy Act (CCPA) in the US. Furthermore, companies themselves will need to reassure patients and participants alike that their privacy is of the utmost importance.
- Incentivizing clinical trials with innovative solutions. By incentivizing clinical trials focusing on the development of innovative solutions, then taking time to address existing unmet needs in various diseases. This can be supported by identifying data gaps in current repositories like an Electronic Health Record (EHR) and claims data, which can be combined with real-world data gathered from mobile apps or wearables. To make sense of this vast amount of data, it can be interpreted using AI, as finding biostatistical methods will be instrumental in driving innovative solutions forward.
- Participating in open platforms. This is a natural progression of the intended shift in clinical research that not only brings researchers, patients and institutes together, but can also serve as hotspots for innovation. For example—VirTrial, a hybrid decentralized virtual platform is set to replace 25-40% of standard clinical trial visits with decentralized clinical visits to create hybrid Phase III and IV studies.
Volumes of new data will be generated now that Covid-19 has impacted clinical trials, and everything has moved to virtual platforms. If big data renaissance, collaboration, bringing out comparative advantages of clinical trials to design niche therapeutics and ensuring uninterrupted real-time flow of harmonized data is brought together, then a digital revolution in clinical research can be orchestrated.