US FDA’s Requirements on CAR-T Therapy
Overview
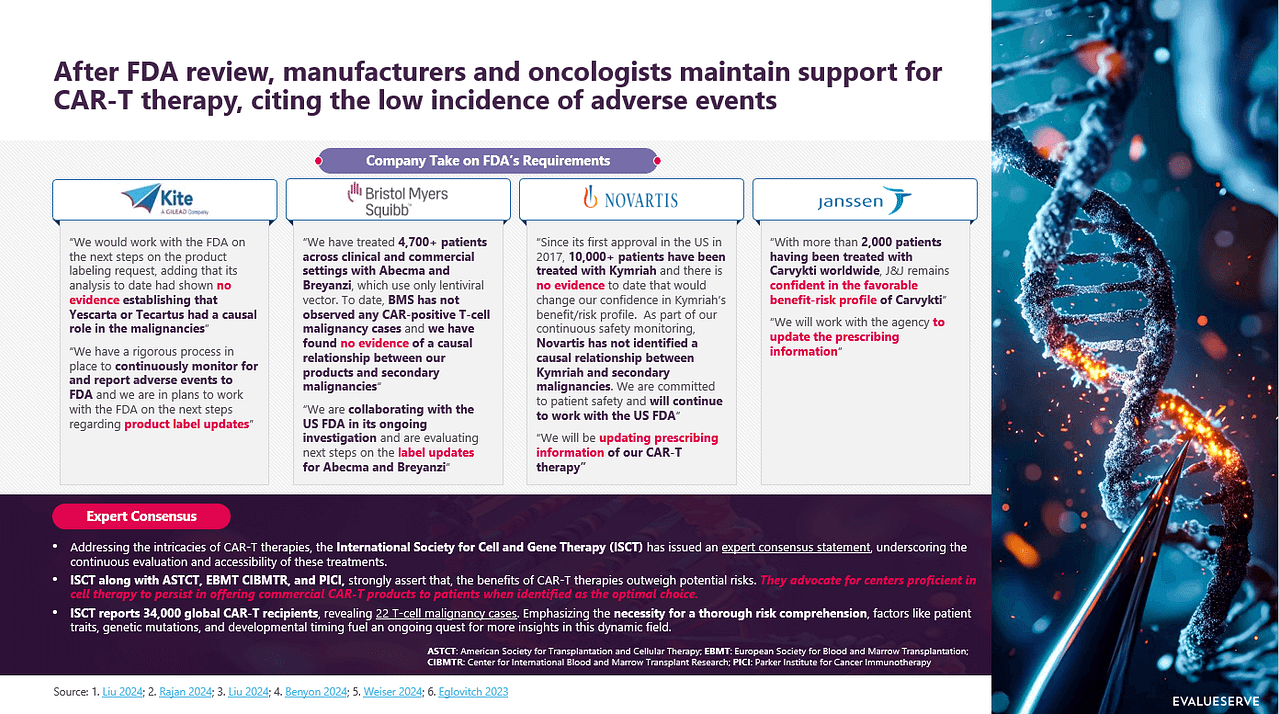
In a proactive effort to prioritize patient safety, the US Food and Drug Administration (FDA) has mandated "black box warnings" for six approved CAR-T cell therapies targeting BCMA and CD19 antigens. These therapies include Yescarta, Tecartus, Carvykti, Abecma, Breyanzi, and Kymriah. This action follows reports of rare T-cell malignancies associated with these treatments. Despite these potential risks, industry stakeholders including associations, key opinion leaders (KOLs), and manufacturers maintain that the benefits of CAR-T therapies outweigh the risks.
In this report, Evalueserve provides a concise overview of the current market landscape, implications, and trends in the CAR-T therapy space.
Contributors

Aditya Singh
Senior Analyst

Vasundra Kar Puri
Principal Consultant
Publication
Download the publication
To know more, please download the full report.